
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
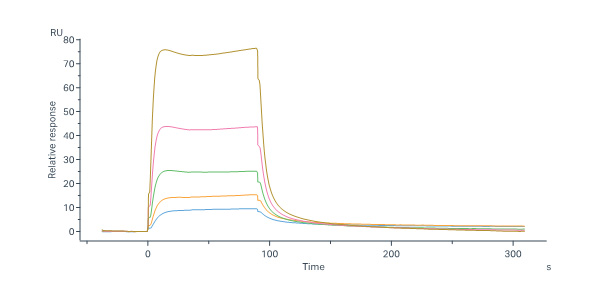
| FG4-H5253 | Human | Human FGF R4 / CD334 Protein, Fc Tag (MALS & SPR verified) |  |
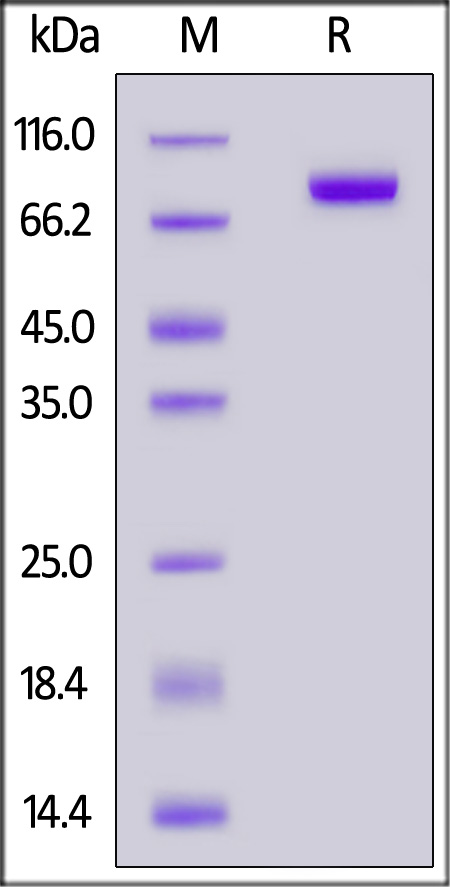
|
|
| FG4-M52Ha | Mouse | Mouse FGF R4 / CD334 Protein, His Tag |  |

|
|
| FG4-H5228 | Human | Human FGF R4 / CD334 Protein, His Tag |  |
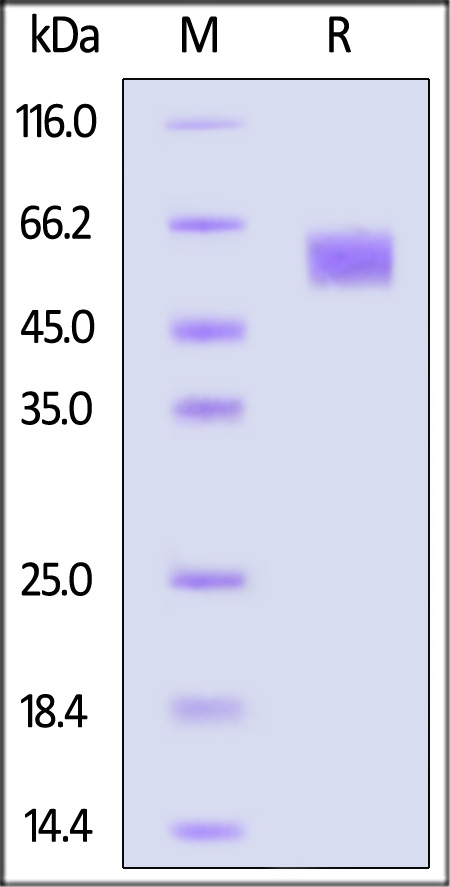
|

Human FGF R4, Fc Tag (Cat. No. FG4-H5253) immobilized on CM5 Chip can bind Human FGF-4 Protein, His Tag (Cat. No. FG4-H51H3) with an affinity constant of 1.48 μM as determined in a SPR assay (Biacore 8K) (QC tested).

The purity of Human FGF R4, Fc Tag (Cat. No. FG4-H5253) is more than 90% and the molecular weight of this protein is around 145-175 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Lenvatinib Mesylate | MK-7902; ER-203492-00; E-7080 | Approved | Eisai Co Ltd | Kisplyx, Lenvima, Lenvima/Kisplyx, 乐卫玛 | United States | Thyroid Neoplasms | Eisai Inc | 2015-02-13 | Neuroendocrine Tumors; Paraganglioma; Thyroid Cancer, Papillary; Melanoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Glioma; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Hepatic Insufficiency; Breast Neoplasms; Cholangiocarcinoma; Osteosarcoma; Solid tumours; Carcinoma, Adenoid Cystic; Adenocarcinoma, Follicular; Liver Diseases; Thyroid Carcinoma, Anaplastic; Adenocarcinoma of Lung; Neoplasms; Renal Insufficiency; Pheochromocytoma; Esophageal Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Liver Neoplasms; Biliary Tract Neoplasms | Details |
| Futibatinib | TAS-120 | Approved | Taiho Pharmaceutical Co Ltd, Otsuka Pharmaceutical Co Ltd | LYTGOBI | United States | Cholangiocarcinoma | Taiho Oncology Inc | 2022-09-30 | Central Nervous System Neoplasms; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis; Endometrial Neoplasms; Lymphoma; Metastatic breast cancer; Sarcoma; Cholangiocarcinoma; Breast Neoplasms; Biliary Tract Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Esophageal Neoplasms; Carcinoma; Stomach Neoplasms; Bone Marrow Neoplasms; Solid tumours | Details |
| Erdafitinib | 890E37NHMV; G-024; JNJ-493; JNJ-42756493; TAR-210 | Approved | Astex Pharmaceuticals Inc | Balversa, 博珂 | United States | Carcinoma, Transitional Cell | Janssen Biotech Inc | 2019-04-12 | Carcinoma, Squamous Cell; Osteosarcoma; Sarcoma, Ewing; Neuroblastoma; Prostatic Neoplasms; Bile Duct Neoplasms; Urologic Neoplasms; Histiocytic Sarcoma; Hepatic Insufficiency; Lymphoma; Lymphoma, Non-Hodgkin; Sarcoma; Glioma; Metastatic breast cancer; Carcinoma, Neuroendocrine; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Neoplasms, Germ Cell and Embryonal; Neuroectodermal Tumors, Primitive, Peripheral; Xanthogranuloma, Juvenile; Adenocarcinoma; Hepatoblastoma; Ependymoma; Medulloblastoma; Rhabdomyosarcoma; Hematologic Neoplasms; Bone metastases; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma; Rhabdoid Tumor; Solid tumours; Glioblastoma; Carcinoma, Transitional Cell; Neoplasms; Wilms Tumor; Multiple Myeloma; Urinary Bladder Neoplasms; Central Nervous System Neoplasms; Prostatic Neoplasms, Castration-Resistant; Histiocytosis, Langerhans-Cell | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Rogaratinib | BAY-1163877 | Phase 3 Clinical | Bayer AG | Solid tumours; Neoplasms; Carcinoma, Transitional Cell; Urinary Bladder Neoplasms; Breast Neoplasms; Sarcoma; Gastrointestinal Stromal Tumors; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung | Details |
| SC-0011 | SC-0011 | Phase 3 Clinical | Shijiazhuang Zhikang Hongren New Drug Development Co Ltd | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| AUR-109 | ODM-203; AUR-109 | Phase 2 Clinical | Orion Corp | Ovarian Neoplasms; Liver Neoplasms; Kidney Neoplasms; Solid tumours; Carcinoma, Renal Cell; Urinary Bladder Neoplasms; Pulmonary Fibrosis; Breast Neoplasms; Colorectal Neoplasms; Lung Neoplasms | Details |
| Fisogatinib | BLU-554; CS-3008 | Phase 2 Clinical | Blueprint Medicines Corp | Carcinoma, Hepatocellular | Details |
| Gunagratinib | ICP-192 | Phase 2 Clinical | Beijing Tiancheng Pharmaceutical Technology Co Ltd | Solid tumours; Biliary Tract Neoplasms; Head and Neck Neoplasms; Stomach Neoplasms; Carcinoma, Transitional Cell; Urinary Bladder Neoplasms; Cholangiocarcinoma; Lung Neoplasms | Details |
| Irpagratinib | ABSK-011 | Phase 2 Clinical | ABbisko Therapeutics Co Ltd | Solid tumours; Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| BPI-43487 | BPI-43487 | Phase 1 Clinical | Betta Pharmaceuticals Co Ltd | Solid tumours; Biliary Tract Neoplasms; Carcinoma, Hepatocellular | Details |
| HS-236 | HS-236 | Phase 1 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Solid tumours | Details |
| HS-10340 | HS-10340 | Phase 1 Clinical | Changzhou Hengbang Pharmaceutical Co Ltd, Shanghai Hansoh Biomedical Co Ltd, Jiangsu Hansoh Pharmaceutical Group Co Ltd | Solid tumours | Details |
| ICP-105 | ICP-105 | Phase 1 Clinical | Nanjing Tianyin Jianhua Pharmaceutical Technology Co Ltd, Beijing Tiancheng Pharmaceutical Technology Co Ltd, Beijing Innocare Pharma Tech Co Ltd | Liver Neoplasms; Solid tumours | Details |
| ZSP-1241 | ZSP-1241 | Phase 1 Clinical | Guangdong Zhongsheng Pharmaceutical Co Ltd, Wuxi Apptec Co Ltd | Liver Neoplasms; Biliary Tract Neoplasms; Stomach Neoplasms; Carcinoma; Esophageal Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Carcinoma, Hepatocellular | Details |
| H3B-6527 | H3B-6527 | Phase 1 Clinical | H3 Biomedicine Inc | Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| SYHX-2005 | SYHX-2005; SYHX2005 | Phase 1 Clinical | Cspc Ouyi Pharmaceutical Co Ltd | Solid tumours | Details |
| JAB-6343 | Phase 1 Clinical | Jacobio Pharmaceuticals Co Ltd | Solid tumours | Details | |
| SY-4798 | SY-4798 | Phase 1 Clinical | Shouyao Holding (Beijing) Co Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| ABSK-012 | ABSK-012; ABSK012 | Phase 1 Clinical | ABbisko Therapeutics Co Ltd | Solid tumours | Details |
| LY-2874455 | LY-2874455 | Phase 1 Clinical | Eli Lilly And Company | Neoplasms; Leukemia, Myeloid, Acute | Details |
This web search service is supported by Google Inc.







